In the realm of lithium battery recycling, the anode and cathode sheets play different roles. As the demand for sustainable resource management grows, understanding the disparities in recycling these two components becomes of utmost importance. This not only aids in optimizing the li-batteries recycling process but also in recovering valuable materials more efficiently. So, what exactly sets the recycling of anode sheets apart from that of cathode sheets? Let’s explore.
Differences in Recycled Object Composition Between Cathode and Anode Sheets
Anode sheet involve graphite and carbon materials
The anode (negative electrode) materials of lithium – ion batteries commonly include carbon materials such as graphite. During the recycling process, the focus is on separating and recycling graphite and other components, as well as the possible copper foil. When recycling, the separation method of graphite and copper foil needs to be considered, such as physical shredding followed by screening to separate the graphite and copper foil.
Cathode sheet contain metal materials
The cathode (positive electrode) materials contain a variety of metal elements such as cobalt, nickel, manganese, and lithium. During recycling, specific chemical leaching processes are required to extract these metals. The equipment must be able to withstand the corrosion of corresponding chemical reagents and effectively separate and enrich metal ions.
Different Recycling Processes and Principles Between Cathode and Anode Sheets
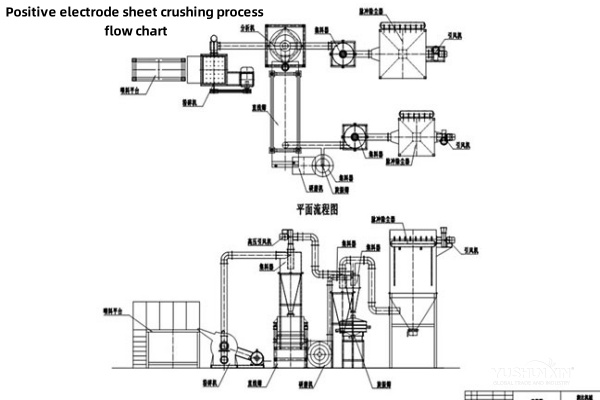
Chemical Recycling is the Key for Cathode – Sheet Recycling Machine
For cathode materials containing cobalt, an acidic leaching agent (such as sulfuric acid) may be used under certain temperature, pressure, and reaction – time conditions to leach cobalt and other metal ions into the solution.
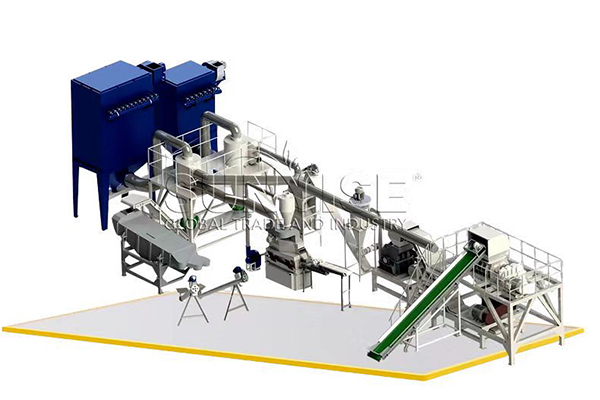
Differences in Equipment Structure and Components Between Cathode and Anode Sheets
Contact Us